CHALLENGING BASIC ASSUMPTIONS: May 25, 2002
INTRODUCTION/ABSTRACT
Many signs today indicate poor growth in multiple marine organisms, which seems to suggest declining total productivity, yet stable levels of bio-available nitrogen in seawater appear to negate the possibility of a systemic nutrient shortage. Is this a valid conclusion? A fundamental similarity between human blood and seawater has been noted for some time. The ratio of concentrations of the major ions is identical in both liquids, for instance. Basic features of seawater and blood, primal life-supporting fluids, appear to be essential to the sustenance of living cells, organisms, and perhaps even systems. In an attempt to illustrate why levels of bio-available nitrogen (hereinafter nitrogen) in seawater would not be expected to drop in initial response to systemic nutrient depletion, an analogy is drawn between nitrogen in marine biological systems and blood glucose in mammalian biological systems (e.g. human). Seawater nitrogen and blood glucose are analogous compounds in their significance and the functional roles assumed in the ‘ocean organism/system’ and the ‘human organism/system’ respectively. The essence of the difference reflects only the difference between autotrophic (e.g. the ocean as a single entity) and heterotrophic (e.g. human) organisms. Functional design principles are essentially the same in many living systems. And that is where the similarity between blood glucose and ocean nitrogen lies. Growth of the marine autotroph is essentially protein limited, while growth of the heterotroph is basically carbon limited…hence the similarity of the roles played by ocean nitrogen and blood glucose. Although chemical differences between the two compounds obviously exist, it is the functional roles played by blood glucose and ocean nitrogen as the basic drivers/controllers of metabolic processes in their respective systems that are highly similar. In each case, the ‘ocean organism’ and the ‘human organism,’ the compounds in question function simultaneously as critical building blocks and as crucial elements in the processes involved in ‘harnessing energy’ or carbon intake and accumulation by the organism. The supply of the key compounds, nitrogen and glucose, determines how much energy, or fixed carbon, is taken in and stored by their respective organisms. And the continued life of both ‘organisms’ depends on the continued availability of these crucial materials. Nitrogen and glucose also must be present in solution in water, and maintained within the tight range of concentration values that is conducive to supporting the continued biological processes of the living systems or ‘organisms.’ Therefore, to maintain optimal functioning, each of these biological systems has evolved with intricate control systems and feedback mechanisms that work to stabilize the availability of the key compounds. And a very high physiological priority has been placed on maintaining ocean nitrogen and blood glucose within normal levels (of both supply and concentration) - in fact, in both cases these will be biologically stabilized by the living system literally "at all costs." In the human example, the complex of physiological mechanisms that act in concert to maintain stable blood glucose levels are now fairly well understood by students of human medicine (although doubtless the complete complexity of the system has yet to be described). Body systems directly involved in maintaining a stable supply and concentration of blood glucose include the digestive, circulatory, endocrine, renal, hepatic and lipid storage systems (fat). Should the blood glucose level rise too high ("hyperglycemia"), immediate physiological reactions occur that tend to lower it. This includes an acceleration in the production of insulin, which hastens the absorption of glucose into somatic cells, along with the prompt excretion of glucose through the urine. On the other hand, if the concentration of blood glucose drops too low ("hypoglycemia"), besides the development of hunger, hormonal processes act to stimulate the release of stored glucose, both from more the quickly accessible short-term storage depot (glycogen) and from the the body’s more long-term energy storage depot (body fat). Biological processes analogous to these also function in the ocean to ensure stability of the supply and concentration of nitrogen in seawater. Briefly (and explored in more detail in the next section of this report), the ocean-system processes that act to compensate for low nitrogen in the photic zone are nitrogen fixation by cyanobacteria, the production of pelagic spawn by many organisms, possibly the vertical migration behavior of zooplankton, and various strategies designed to remobilize nitrogen that is being held in temporary storage depots. The ‘storage depots’ for nitrogen in the sea are living organisms, therefore ‘remobilization’ strategies necessarily include the killing of higher animals…starvation, plus the production of toxins specifically designed to kill these creatures, are the processes that accomplish this end. Interestingly…it can be seen that the lower, smaller creatures, such as the legions of small marine invertebrates, are not vulnerable to the marine biotoxins. Why not? This seems most likely to be because of their major role as stabilizers of the zooplankton (which is analogous to the vital cellular component of human blood). And how do invertebrates stabilize zooplankton? By the production of prodigious numbers of floating spawn, marine bivalves, crustaceans, echinoderms and others are major contributors to the zooplankton, and thereby also to the stability of nitrogen availability in the photic zone. This class of marine organisms can therefore be perceived to be roughly as important to the ocean as bone marrow is to the human (…and bone marrow will not be liquidated to maintain basic metabolism until the very last stage of the starvation process). Spawn production by benthic invertebrates is virtually equivalent to straight ‘zooplankton production’ due to the high mortality rates normally experienced by the pelagic young of all spawning species. Invertebrates do not produce all of the marine spawn in the ocean, however. Fairly high numbers of spawn are also produced by the majority of bony fishes.…and one role that they play in the systemic effort to compensate for lowered nitrogen availability, appears to be an increase in the priority placed on spawn production by these fish. Today we see a widespread trend of declining age and size at maturity in marine fish, as the ocean increases her efforts to stabilize the composition of her ‘blood,’ which is the seawater of the photic zone. Roe is forming in much smaller fish now than was ever observed by biologists in the past. (Godo and Haug, Lilly et al, 1999, DFO, 2000a) Greater numbers of floating eggs and pelagic larvae have the effect of increasing the nitrogen supply in upper regions of the water column. Therefore, the decrease in age and size at maturity of fish can plausibly be hypothesized to be a compensatory reaction of the ‘ocean organism’ to a declining availability of nitrogen The timing of spawn production is also noteworthy. Spawning, when all species are considered, covers the entire year but occurs at a very high rate during spring, summer and fall. This timing helps the ocean system to maximize the opportunity for photosynthesis when light availability is greatest, and also helps to ward off severe nutrient depletion of surface waters in summer. As with the example of blood glucose, the other important aspect in the maintenance of ocean nitrogen levels is having strategies in place for the avoidance of nitrogen concentrations that are too high. Examples of this process, the method by which the ocean lowers the nitrogen level, are easily observed today in sewage polluted estuaries. Termed "eutrophication," the sequence of events includes a heavy bloom of phytoplankton, which acts to absorb the nitrogen from the water, followed by the sinking of the dead algae to the bottom, where bacterial decomposition takes place. Oxygen is consumed by the decomposition process, which then may induce the development of a degree of hypoxia in bottom water. Low oxygen concentrations act to accelerate the action of denitrifying bacteria, which work to rid the system of the excess nitrogen by converting it back to the atmospheric form, gaseous N2. In the ocean, therefore, the problem of excess seawater nitrogen is handled quite effectively on a local scale, as compensatory mechanisms return the values to normal. There are very localized coastal areas where this compensatory mechanism for high seawater nitrogen has been overwhelmed by the constant high input (anthropogenic runoff) and nitrogen levels in the water remain high, but in the vast majority of marine areas seawater nitrogen levels are effectively held the within normal range for ocean health. There appears to be a lot more evidence today that the ocean is relying heavily on her ‘nitrogen raising’ strategies rather than on her ‘nitrogen lowering’ strategies. Viewing the marine ecosystem in this manner may help to provide a clearer insight into the relationship between the concentration of nitrogen in seawater and total marine productivity. There is no expected relationship, since the maintenance of stable nitrogen levels will be done by the living system "at all costs." Just as measurements of blood glucose in humans reveals no information regarding how much energy is being expended, nor whether the person is gaining or losing weight, measurements of seawater nitrogen concentrations are of no use in estimating rates of marine primary productivity, or of the magnitude or direction of the net flow of organic nitrogen. An assumption of a constant rate of marine carbon flux (as has been made) simply cannot be based on the observation of constant nitrogen levels in seawater. And this same argument (with a few more twists) can be applied to the utility for this purpose of measurements of chlorophyll concentration. Unfortunately for those trying to gauge the health of the ocean, measurements of nitrate and chlorophyll concentrations in seawater are essentially all of the "nutrient"-related data that they have, that extends over any significant length of time. (There exists about four decades of this data from various area in the North Atlantic, for example, which reveals a general stability in nitrate levels over that term. (e.g. DFO, 2001)) In recent decades marine scientists have come to appreciate the limitations of this data and have devised methods for recording the actual rate of carbon-uptake by phytoplankton. Many areas of the world ocean have been sampled and measurements have been obtained of "grams of carbon fixed per cubic (or square) meter per day." This provides ‘snapshots’ of the primary productivity of different areas, but this data - although laboriously obtained - also has limited usefulness for the purpose of my particular inquiry: determining whether or not centuries of fishing has already lowered the rate of marine productivity. One weakness in this approach is the short time-span over which the rate measurements are taken - it is generally about one day. The other essential thing is to collect time-series data on primary production. A program to do that has lately been part of an international effort by scientists to monitor the health of the marine ecosystem (GLOBEC). Beginning in 1988, repeated measurements of primary production rates have been obtained at two stations, one at Bermuda and the other in Hawaii. These are generating the first time-series of data on phytoplankton primary productivity. The trends revealed to date are of interannual fluctuations but no clear trend has emerged yet. (Bates, 2001) One weakness in the method used (C14 uptake) is that carbon taken up by cyanobacteria (N-fixers) is not distinguished from that taken up by algae, so that the relationship between nitrogen cycling patterns and productivity is difficult to determine. The carbon flux of an organism can be estimated by a variety of other observations, however, and this applies equally to the human and the ocean systems. For a human, although it’s not a completely straightforward relationship, carbon balance can be approximated by the quantity of body fat that has accumulated. Fat is the primary organ that functions as a carbon depot. (Factors such as environmental temperature, metabolic rate and activity level have an impact on the amount of stored body fat, but a basic principle is well known: individuals taking in larger amounts of carbon (food) will have significantly greater fat stores than those who eat less.) A look at which parts of the ocean system constitute the ‘fat,’ and trends over time in this particular component of the ecosystem, will reveal important information about long-term trends in marine primary productivity. (In the ocean system, a functional autotroph, nitrogen storage will be roughly analogous to carbon storage in the heterotrophic human.) The remainder of this report will examine various aspects of marine ecological processes from the standpoint of their roles in maintaining the stability of nitrogen availability in seawater. Parallels between functional parts of the living marine system and the roles played by different human body systems in glucose metabolism, suggest an alternate view of how changes in the marine ecosystem may be interpreted in the assessment of the overall "health" of the ocean. This also suggests a means to estimate trends in marine carbon flux over a longer term - whether the ocean has been maintaining a constant productivity or alternately has been "losing weight." Physiological changes in a starving organism are compared to today’s broad changing trends in marine life to gain insight into whether or not "the ocean is starving."
A system of complementary control mechanisms exists, along with feedback patterns that switch these off and on as needed, the net effect of which is the maintenance of optimal nitrogen availability in seawater to sustain continued production of marine life. (1) Biological processes that act to lower seawater nitrogen levels. These are analogous to the ordinary metabolic processes that consume glucose and prevent the accumulation of excess concentration of this chemical in human blood. First, it is obvious that the growth of phytoplankton involves the uptake of nitrogen from seawater for use in the growth of the plant. This has an effect of lowering the nitrogen concentration, and is analogous to the constant consumption of blood sugar by the ordinary cell processes of living human metabolism. There is no significant negative feedback working at this point: available nitrogen in the photic zone will simply be taken up by the metabolism of phytoplankton, in the same way that glucose, once it is within somatic cells, will be completely metabolized. On that scale, the reaction only moves in the one direction. An important point regarding nitrogen in the sea is that ammonia (constantly excreted through the gills of all fish and zooplankton) is taken up by phytoplankton much more efficiently than are other nitrogenous forms, such as nitrate. (Lobban and Harrison, 1994) Therefore, ammonia will not accumulate to any extent in surface waters due to the rapidity of uptake, and the quantity of N-cycling mediated in this way can not be accurately determined with any type of instantaneous measurements. The second process that acts in the ocean to lower nitrogen in the photic zone can be summed up as the ultimate tendency for organic particles to sink in seawater. Dissolved nitrogen itself has no sinking tendency, but as soon as it is incorporated into the forms of tiny algae (or bacteria) or things larger, the tendency towards sinking becomes a major force. Growing by photosynthesis, phytoplankton accumulate mass and may sink as a result, or they are consumed by zooplankton, who, besides excreting the ideal N-fertilizer for phytoplankton (ammonia) which stimulates more growth, also excrete sinking fecal pellets. Bacteria grow in clumps on particles of detritus which also sinks. The net effect of this is a "rain" of organic matter that constantly falls from the photic zone to the seabed. The effect of this sinking tendency on nutrient cycling is relatively well understood and described in the marine biology literature. A third process that acts to lower the concentration of nitrogen in seawater is denitrification. Denitrification has the opposite effect of nitrogen fixation, and is ordinarily not a major feature of marine nutrient cycling - it’s a process that is triggered only when the nitrogen concentration becomes too high (in waters affected by eutrophication, and to some extent in the seabed). Denitrification is analogous to an excretory function that is used only when needed; in the human analogy the comparable process is the excretion of sugar in the urine. When the system is functioning in normal health, insignificant amounts of sugar are excreted in urine, but when hyperglycemia develops, excretion of sugar in the urine begins immediately and can reach high proportions as the body seeks to maintain stability of the immediate concentration of dissolved sugar in the blood. (2) What forces act to complement the nitrogen-lowering processes, how does the ocean system manage to replenish nitrogen in the surface water? For nutrient cycling to be continuous in the ocean, there must be equivalent physical forces that counteract the constant sinking tendency of organic matter. Which forces are involved in refreshing the nitrogen supply in the surface waters? The best understood method by which nitrogen is returned to the surface water is through forces that lead to physical mixing of the water column. This is not a biological process, but the living system has evolved to co-ordinate its efforts to use this natural phenomenon to full advantage. Seasonal cooling of the surface in temperate zones as well as wave action, mix the richer bottom layers with the upper, refreshing the potential for photosynthesis in the surface layer through nitrate fertilization. Driven solely by annual weather patterns, this is termed "physical forcing" and scientific recognition of this process has led to a perception that weather patterns alone determine the rate of biological production in the ocean. There is a vast reservoir of unavailable nitrate in the deep ocean and on average, if weather patterns are constant, it stands to reason that the same amount of this will be forced into the photic zone each year. If the physical water movements resulting from weather patterns actually do control the availability of nitrogen (as nitrate) in the photic zone, then the assumption of a steady rate of marine carbon fixation (as long as weather patterns are reasonably constant) is reasonable. There are, however, biological processes that actively work to return nitrogen to the surface waters, including processes that directly counteracting sinking, and these processes also therefore affect the total rate of primary productivity that occurs. In a more robust biological system these effects will predictably be stronger, leading to the conclusion that the absolute rate of marine carbon fixation is linked not only to "physical forcing" but also to the number of functioning animate marine life forms in the sea. First, it is worth noting, and has long been included in marine literature, that the consumers of phytoplankton, the zooplankton, cycle nitrogen repeatedly through the phytoplankton and thereby stimulate a greater level of net primary productivity than what would occur in their absence. (e.g. Kerfoot and Sih, 1987) While this does not initially appear to entail an effect to counteract sinking, it is the first biological function that acts to enhance primary production. Since zooplankton growth, termed "secondary production," has been envisaged as resulting as a direct consequence of "primary production," this fertilizing effect of the zooplankton has been also assumed to be, at its basis, also "physically forced." This finding of cycling mediated by zooplankton apparently has not changed the basic view that net system productivity is physically forced because the cycling involved has been observed to occur within the photic zone. However a few facts about zooplankton would seem to complicate the picture, and suggest that zooplankton may be involved directly in "biological forcing" of production. The first observation is the fact that zooplankton do not stay in the photic zone. They are well known to undertake long, daily vertical migrations into the deeper, darker parts of the water column. Tiny individual organisms may sink 50 meters or more during the day and then swim back up to the surface waters each night. The exact reasons for this behavior pattern remain a source of debate among scientists. (Steidinger and Walker, 1984) The vertical migration behavior appears to be energetically wasteful. But another detail of zooplankton physiology may offer a clue as to one unsuspected reason that might underlie the migratory behavior. Some zooplankton species, notably the highly abundant copepods, appear to have the ability to uptake dissolved organic material directly from the water. (Steidinger and Walker, 1984) Besides moving between high-light to low-light parts of the water column, zooplankton are also moving between areas of lower and higher concentration of dissolved nutrients during their daily migrations. This raises the intriguing hypothesis that they may absorb nutrients directly from the deeper water and raise those nutrients to the surface, where they may then be released to amplify the fertilization of the phytoplankton. Zooplankton may also feed on detritus in the deeper water and raise nutrients to the surface as a result of that activity. Zooplankton might be visualized as a horde of little 'worker bees' that continually pull organic nutrients from the depths toward the surface of the sea...a part of the overall pattern designed specifically to counteract the sinking of organic particles. In summary therefore, nutrient cycling mediated by zooplankton cannot be considered to be purely a process that occurs within the photic zone, and is therefore "physically forced"...because the zooplankton do not physically stay at the top of the water column. If the vertical migratory behavior of zooplankton accomplishes a net shift of nitrogen, for instance, from lower to higher levels in the water column, it also bears consideration whether or not this may also be a feature of similar migratory behavior by some larger species, such as shrimp. Notably absent from current analyses of marine ecological processes is an appreciation of one major biological process that unarguably does act to counteract the physical sinking tendency of organic matter and actively regenerates bioavailable nitrogen in the photic zone from the seabed. This process is the production of pelagic eggs and spawn. Most marine spawn floats, and eggs and larvae released below the lower limits of the photic zone can commonly be seen to float up into it. Important organisms involved in this process include the bottom dwelling fish, but the greatest contributors are the benthic invertebrates. This biological function, spawning, actively transports N-rich organic material from the lower water column back up into the photic zone. Benthic organisms that feed low in the food web, capitalizing on sunken phytoplankton or other detritus, play a major role in forcing the return of organic nitrogen to the surface waters through the release of their floating spawn. Many species, including bivalves and echinoderms, can be perceived as devices that function to take in sunken phytoplankton and detritus and pump out floating zooplankton. Individual organisms commonly release millions of gametes. (Kasyanov, 2001) This is a slightly indirect, but very real, route by which phytoplankton growth stimulates the production of zooplankton, and does not fit the classic concept of the "secondary production" pathway because there is an essential ‘middle man’ in the picture, in the form of the mature benthic spawning organism. This is a quicker and more direct route by which nitrogen is returned to the photic zone, than is the classic picture of sinking organic material undergoing bacterial decomposition with the release of dissolved nitrate into the bottom of the water column, eventually to be returned to the surface by "physical forcing" triggered by weather patterns. How does the pelagic spawn of benthic organisms act to replenish nitrogen in surface waters? First, by virtue of their normal metabolic processes as already discussed, even if they consume nothing, all of these tiny organisms are ‘slow release’ devices for ammonia. Secondly, even though they are only temporarily resident in the zooplankton, many will engage in feeding on phytoplankton and recycling nitrogen in place, contributing to the stimulation of greater levels of primary productivity. Thirdly, these tiny eggs and larvae are frequently consumed by the major species that constitute the full-time zooplankton, such as copepods and chaetognaths, enhancing their growth and fertility, all of which enhances the net rate of primary production by the phytoplankton. The pelagic spawn of non-pelagic adults, when considered in total, makes up a very significant fraction (sometimes the major fraction) of the total marine zooplankton biomass in neritic waters. (Zhong et al, 1989) "Secondary production" (aka "zooplankton production") is therefore clearly not only linked to nitrate-driven primary production, but also to the size of the total standing stock of pelagic spawn producers. Humans have tended to view the development of the adult stage of these species as the "goal" of their spawn production. This is merely a human bias, however, perhaps colored partly by our desire to eat the adults produced, and also by our perception that the goal of "reproductive strategies" of animals must be analogous to ours, i.e. the survival of a maximum number of offspring to maturity. But overwhelmingly, these invertebrates and fish "fail" to produce more than miniscule "survival" percentages in their offspring, as the large majority die during the pelagic life stages. This then, must be the primary goal of the spawning behavior, effectively the production of zooplankton and the physical raising of nitrogen-rich organic material from the seabed back up into the photic zone. The production of pelagic spawn by marine organisms, fish and invertebrates both, therefore constitutes a ‘biological upwelling’ of nutrients in the coastal ocean, which effectively enhances "secondary" production as well as "primary" production. This appears to be one part of the intricately woven web of sea life that was designed to counteract the tendency of organic particles to sink in a liquid medium, a necessity in a growing medium where the sun shines only on the surface. Mother Ocean, you see, has long known how to deal with the gravity of the situation… Practically all marine species of fish and invertebrates will be classified as zooplankton at some point in their lives. And when they do, they all occupy the same "ecological niche" and perform the same "ecological role." The role played at that stage is providing food for plankton-feeders as well as stimulating an acceleration of primary production. For many marine species it is essential that zooplankton be available for consumption, at least during the early life stages. Phytoplankton alone will sustain very few of them. A very high priority is therefore placed on the maintenance of a steady supply of zooplankton in the ocean. The importance of zooplankton in ‘living’ seawater can be seen to be roughly equivalent to the importance of the tiny blood cells that float in the liquid medium on which human life depends. As with the concentration of nitrogen or glucose, the next order of importance in maintaining healthy ‘lifeblood’ is the concentration of the tiny individual blood cells or the tiny animal forms in the sea, the zooplankton. Producers of pelagic spawn therefore, especially the top producing invertebrates, many bivalves and echinoderms, are of a more critical importance in maintaining ecosystem stability than are species groups that play less central roles in this aspect of nutrient cycling. Like the bone marrow, which produces our primary blood cells, these lowly invertebrates will be maintained for a longer time under systemic starvation than will organisms whose roles are more analogous to our body fat. Therefore, when the ecosystem is forced to downsize and shift a greater fraction of its reserves into the maintenance of the essential constituents of its ‘lifeblood,’ invertebrates such as these will initially be spared. How does the ocean mobilize its nitrogen reserves when this becomes a necessity? Analogous to a starving person remobilizing stored energy reserves, some of the tissue that performed the storage role will be broken down and the constituent organic material shifted to places where it can be re-used to replenish the essential ‘blood components,’ dissolved nitrogen and zooplankton. A shift in the metabolic pattern occurs…the human may reverse the net process, going from gaining weight to losing weight, but the internal metabolism will adjust automatically to constantly maintain healthy blood. There appears to be two major biological functions in the sea which act to remobilize stored nitrogen for use in seawater maintenance. These are the production of biotoxins and starvation. The production of biotoxins by marine phytoplankton appears rather inexplicable to us at first glance, but a look at some of the details makes a plausible argument that the production of these biotoxins is a system response to the general lowering of the availability of dissolved nutrients, bio-available nitrogen as well as phosphorus. Biotoxins are produced by a variety of photosynthetic organisms across all major groups of these - diatoms, dinoflagellates, cyanobacteria, members of all these groups have been known to produce biotoxins under the ‘right’ conditions. (Lassus et al, 1995) The most troublesome of these tend to be cyanobacteria and dinoflagellates, and their toxicity to humans along with their increasing prevalence in the world ocean, have drawn a fair amount of scientific attention to the subject. The organisms that produce the biotoxins are typically smaller than other types of phytoplankton, and are consistently known to bloom under weather conditions that promote extreme levels of nutrient depletion in surface waters (warm, calm spells: this type of weather very consistently precedes blooms of toxin-producing phytoplankton, whether near shore or offshore, dinoflagellates or cyanobacteria. (Lassus et al, 1995)) These types of plankton can be difficult to culture in laboratory conditions, the cyanobacteria in particular will grow only in extremely N-depleted media. This seems to be the necessary condition to get the growth started, and when nitrogen levels start to rise, cyanobacterial growth is inhibited. (Lassus et al, 1995) Cyanobacteria are the only class of marine algae that can accomplish N-fixation and, probably for this reason, they have long been known to be more prominent in oligotrophic systems. An interesting detail of toxic cyanobacterial culture is that the concentration of the toxin produced can be quite variable. Very low available nitrogen is the first prerequisite for their growth, but as phosphate limitation increases also, the toxin production intensifies. (Lassus et al, 1995) When biotoxin production is viewed as a systemic strategy to remobilize basic nutrients that are tied up in larger marine organisms, this seems to make sense. As the nutrient availability becomes ever lower, the system increases the activity of the homeostatic mechanism designed to counteract the state of extreme depletion: biotoxin production. This natural killing of larger marine organisms such as fish, mammals and seabirds, will tend to replenish both dissolved nitrogen, phosphate and zooplankton production, since the corpses will normally be consumed by benthic scavengers (like echinoderms, extraordinary zooplankton rebuilders) or decomposed by bacteria which results in the liberation of the basic nutrients in dissolved form into the seawater. Marine biotoxin formation under low ambient nitrogen concentrations can therefore be seen as analogous to the hormones released in mammals when blood glucose concentration starts to drop - in our case glucagon is released by the pancreas which causes a prompt release of carbohydrates stored in the liver, and an increase in blood glucose to the normal level. Homeostatic mechanisms, both of these. Interestingly, marine invertebrates are immune to the lethal effects of marine biotoxins, although they are the major consumers of phytoplankton. In fact, lowly invertebrates often act as the vehicle of transmission of the toxins to organisms that feed at higher trophic levels…and this reflects the relative importance of maintaining each of these populations in the ocean. The second route by which the ocean ecosystem remobilizes nitrogen bound in temporary storage is accomplished by the selective starvation of the larger individuals within longer lived, larger species. This is manifested by an increasing contraction of the age structure of multiple fish stocks, and can also sometimes be seen in the declining physical health and condition of the oldest remaining individuals. As with deaths by biotoxins, these natural starvation deaths result in the nutrients stored within the corpses becoming available for cycling at lower levels, thus contributing to homeostasis of the plankton. Analogous to body fat on the human body, the existence of a substantial number of large living fish amounts to a safety margin, a reserve that can be tapped as needed to sustain the essential process that keeps the very system alive, the maintenance of the normal biochemical constituency and "cell counts" of seawater. A biological entity, whether a single human or the world ocean, that possesses just the bare minimum of reserves (i.e. is very thin) is much less resilient to stresses that will naturally be experienced in the environment. The person with no fat reserves will continue to maintain normal blood chemistry and blood cell counts until the starvation problem becomes very acute…and potentially terminal. This is also true for the dissolved nitrogen and basic plankton parameters in a ‘starving ocean.’ Homeostatic mechanisms work to ensure consistency in the makeup of the ‘lifeblood’ in each case until the point of total system collapse or "decompensation." A few more comments on "fat": Beyond functioning as a basic energy reserve in the human, body fat is a dynamic living tissue that offers other positive benefits to the organism. In the human example, fat acts as a thermal insulator and is also involved in complex hormonal processes. The complete physiological significance of body fat is not yet understood. This undoubtedly also applies to the ocean’s equivalent to ‘fat,’ which to a significant extent consisted of large spawning fish. The biggest, oldest spawners grew only very slowly, yet they produced the greatest numbers of fish eggs, and also the greatest mass of spawn by comparison to their own body size ("GSI"). Therefore, the largest fish were more intensely involved in cycling nutrients directly into the zooplankton in the form of their spawn. However, in leaner times these have become too expensive to maintain, and they have been ‘liquidated’ by starvation and the responsibility for zooplankton production placed more heavily on the "lower" bottom-dwelling invertebrates which function more efficiently for the purpose. The largest spawning fish were often observed to follow the longest migratory routes in some species. And it is likely that migratory behavior of fish has also played an important, if subtle, role in marine nutrient cycling patterns…maybe another bit of the complexity of interdependency that we have failed to appreciate…
(* The signs, symptoms and diagnostic tests discussed in this section do not indicate the root cause of systemic starvation, but just help to confirm the presence of the condition. Examining the history of factors that have impacted on the ocean will help to establish cause.) Based on the model of a starving aquatic population (Powell et al, 1995) and the preceding discussion on homeostatic mechanisms that stabilize the supply of nitrogen in seawater, it is suggested that observing the following signs, symptoms, and laboratory findings will be useful in screening for the possibility of systemic starvation in large marine ecosystems. Also discussed are the key points leading to differential diagnosis between systemic nutrient loss and other possible pathologies. I - ESSENTIALS OF DIAGNOSIS: (1) A decline in the maximum sizes of fish. From the model of starvation it is clear that this is the only early warning sign. Since this effect is also caused by fishing, examining unfished populations for this trend is necessary. Steadily declining sizes in unfished populations represent the nearest possible indicator to a "gold standard" sign of declining marine primary productivity. (Also, the maximum size obtainable by individual fish in unfished populations therefore represents a reasonably useful index of total production.) Regarding the cause of a decline in the maximum size of fish, this must be differentiated from overfishing. The key to the differential diagnosis between systemic starvation (food limitation) and overfishing is the condition factor of the oldest living age cohorts. In starvation, this will be observed to decline, while in classic "overfishing" alone, the condition factor in these fish will remain stable or increase. Changes in growth rates have often been seen to reflect similar changes in condition factor. Declining growth rates and declining condition factors both suggest increasing food limitation, but condition factor is the better test since it is much less likely to be under genetic control than is growth rate. (Genetic change has been suggested, although never proven, as a possible cause of slowed growth in heavily fished populations. Condition factor is also affected by "density dependent" effects however, so declining condition in the context of low abundance is the strongest signal of starvation.) (2) A "down the web" shift across species affecting community composition will also be predicted as the overall scope of the ecosystem contracts. This signal can also be confused with the effects of overfishing, as in the model. A shift toward smaller organisms, organisms with lower nutrient requirements and more primitive species will be expected since these were the organisms that originally survived in the young ocean, millions of years ago, during a time when the total marine organic accumulation was less and the rate of nutrient cycling in the sea was therefore also lower. Therefore, be alert for a rising presence of cyanobacteria, jellyfish and crustaceans in the ocean. (3) Records of declining rates of productivity may be discernible in individual members of long-lived species. For example, the baleen of the bowhead whale offers a useful record, as has been documented by one scientist who determined from analyzing bowhead baleen that productivity in the Bering Sea has declined steadily since at least the mid 1960s. (Schell,D.) Many other organisms may prove to be similarly useful for this purpose. (4) Shifting ranges and growth rates in intertidal organisms, especially visible in sessile perennial forms, will reflect the adjustments of these species to the declining availability of nutrients. (See articles on this website outlining changes in barnacles and seaweed.) The exact range shifts will be determined by the natural gradients of food availability in the environments of these organisms. Similar range shifts, contracting toward the areas offering relatively better feeding opportunities, may also be seen in mobile marine species. (5) An increasing prevalence of cyanobacteria will be noted, planktonic and benthic forms both. Accelerated nitrogen fixation is a normal homeostatic mechanism that occurs in response to lowered systemic nitrogen availability. Under ‘systemic starvation,’ this activity will be significantly increased. (6) Laboratory testing will indicate an increase in the biotoxin content of seawater, reflecting the acceleration of another homeostatic mechanism designed to correct for nutrient deficiency in the system. Biotoxins produced in the open ocean more convincingly suggest systemic nutrient loss. Those occurring in polluted estuaries may be partially stimulated by alterations in N:P ratios. (7) A decrease in the age and size of marine organisms that produce pelagic eggs and larvae. There is no proof of this, but it is hypothesized that in a system designed to maintain stable conditions for life, that plasticity of age at maturity in these species might function to stabilize the supply and abundance of zooplankton. If so, declining age at maturity would be expected to accompany a decline in zooplankton abundance and the signal will be found across all spawning species, regardless of the intensity of their fishing histories. (8) A rise may be detected in the concentration of atmospheric CO2. (Shaffer, 1993) (For elaboration on this point see: "Strangelove Ocean") ** THESE NEXT THREE SIGNS ARE MORE SERIOUS -- If these signs occur, they indicate the occurrence of "decompensation," a condition where the activation of the normal compensatory mechanisms has failed to maintain stability within the system. At this point, many things may unexpectedly go awry. (An analogy from human medicine would be the development of "shock" symptoms, which indicate a severely fragile state of health, and one which does not last long. The "shock" patient either receives prompt, appropriate treatment and recovers, or dies.) (9) Decline in the stability of the biochemical makeup of seawater. As biological control mechanisms fail, the nutrient pulses triggered by physical forcing patterns will become exaggerated. In the North Atlantic ocean, for instance, the springtime high nutrient levels may reach greater concentrations and be lowered more slowly by a diminished food web. Similarly, the normal summertime lows will be exaggerated and dip to lower levels. Chlorophyll concentrations will also roughly follow this pattern. An increase in the intensity and duration of the spring phytoplankton bloom, as measured by chlorophyll content, is therefore not an indication of good health and "high productivity" in the ocean, but rather the reverse. (10) A decline in the abundance of zooplankton. A significant drop in zooplankton is a very grave sign, as this component is vital to sea life. Rather than representing the second stage of a production pathway ("secondary production"), zooplankton more correctly represents the essence of marine animal life. (11) A decline in unexploited lower non-planktonic organisms. Declines in organisms such as benthic invertebrates, large populations of which are critical for the maintenance of zooplankton numbers and the rapid recycling of bottom organic matter back to the photic zone, will eventually occur. In the earlier stages of system decline, however, the abundance of these forms may increase as a greater proportion of phytoplankton production sinks to the bottom in the absence of the previous level of zooplankton grazing. If these benthic populations are seen to follow the pattern predicted by the starvation model for oysters, however, and have reached the point of rapidly declining numbers, this suggests that the condition of systemic starvation is at an advanced stage. II. MAJOR CONSIDERATIONS IN THE DIFFERENTIAL DIAGNOSIS OF SYSTEMIC STARVATION IN THE OCEAN (1) OVERFISHING Besides checking the condition factor and growth rates of the oldest individuals, and looking for similar changes in co-existing unfished species, points which have already been discussed, it bears mentioning which diagnostic tests are of very limited or no usefulness in distinguishing between overfishing and systemic starvation. These include assessments of "total biomass," "spawning stock biomass," the abundance of juveniles, and condition factors or growth rates averaged over entire populations. None of these parameters will reliably change during the early stages of population starvation. They will all decline precipitously in the latest stages, however. (2) POLLUTION - CHEMICAL This should usually show more intense effects closest to the pollution source, except for the longest acting pollutants. Beyond this, although it would be expected to sicken or kill individual organisms, chemical pollution would not normally be expected to trigger the onset of gradual changes across the entire ecosystem that are consistent with a decline in overall system productivity. Chemical pollution is generally not considered to have had any significant impact on marine life in the open ocean. (3) POLLUTION - NUTRIENT Excessive nutrient loading of estuaries and coastal waters has increasingly become a focus of concern in marine science, but differentiating between systemic starvation and nutrient overload can be surprisingly difficult. A cluster of signs has been associated with intense eutrophication: elevated dissolved nutrient levels, elevated chlorophyll levels, increased turbidity, hypoxic/anoxic bottom waters, increased dominance of filter feeders, decreased growth of larger, perennial macroalgae, and increased growth of smaller, short-lived macroalgae. (Schramm and Nienhuis, 1996) Some of these diagnostic tests have more value than others, but, unfortunately it seems too often as if the appearance of any one of them is taken as conclusive evidence that "eutrophication" is occurring. And in some situations contradictory signals exist, such as increased short-lived macroalgae accompanied by a decline in filter feeders. A critical look needs to be taken at each of these diagnostic tests for eutrophication to determine its accuracy and usefulness in correctly identifying instances of true nutrient overload of seawater. The "specificity" and "sensitivity" of each test needs to be considered. This is the manner in which the usefulness of medical diagnostic tests is determined. The "gold standard" test, if it exists, is 100% specific and 100% sensitive to the condition being tested for. Tests with high sensitivity but low specificity will give many false positive results, while tests with high specificity and low sensitivity will give many false negatives. Neither of these extremes represents a useful diagnostic test. (a) Elevated dissolved nutrient levels. - This test is very sensitive to eutrophication but may also sometimes test positive in systemic starvation as the ability of the living web to dampen natural nutrient pulses is gradually lost. Therefore specificity of this test is not perfect. Extremely high readings however, are most likely true reflections of eutrophication, while smaller degrees of elevation remain questionable. (b) Elevated chlorophyll levels. - This test is also highly sensitive to eutrophication (occurs in all grossly obvious cases), but similar criticisms apply to chlorophyll levels as to nutrient levels. In a starving system that experiences higher and more prolonged dissolved nutrient pulses from physical forcing, elevated chlorophyll levels would be expected to follow. (Note the increasing intensity of "greeness" of the North Atlantic spring phytoplankton bloom (DFO, 2000, SSR G3-02)…by no stretch of the imagination can this be considered a reflection of "eutrophication.") Another factor that weakens the usefulness of chlorophyll levels is the fact that not only all forms of algae, but also cyanobacteria use this pigment. Cyanobacteria thrive when dissolved nitrogen is low and can ‘self-fertilize’ by nitrogen fixation. Therefore the simple presence of a particular level of chlorophyll does not accurately reflect levels of nutrient input from terrestrial sources. (Note: increasing levels of chlorophyll have been noted over Australia’s Great Barrier Reef - and this is known to be the result of the increasing abundance of Trichodesmium in the seawater. Trichodesmium is a common marine planktonic form of cyanobacteria, all of which grow best in nitrogen-depleted media.) Therefore simply looking at chlorophyll levels is a very poor test for nutrient overload of seawater. It may be a helpful indicator if used with other tests, but is unreliable if used alone. (c ) Increased turbidity/decreased light penetration (lowered secchi disk values). This test is highly sensitive to eutrophication and should rarely occur in marine systemic starvation, with the possible exception of during seasonally forced spikes in nutrient and chlorophyll levels, if these become intense. If this tests positive throughout the entire year, however, it becomes more specific for the detection of true cases of eutrophication. (d) Hypoxia or anoxia of the bottom layer of the water column. Again, this test is highly sensitive for severe eutrophication, but may occasionally give a false positive in the instance of a severely starved ecosystem in which the living web can no longer effectively absorb seasonal natural nutrient pulses. The natural nutrient pulse could result in a heavy bloom of phytoplankton which, if not processed by a normal zooplankton assemblage, might form a heavy enough deposit on the seabed that bacterial decomposition might induce hypoxia. (Note: An incident that occurred off Africa early in 2002 may represent an example of this type of scenario. Nutrients raised by natural upwelling currents triggered an intense diatom bloom, which fell to the bottom in such quantity that oxygen-depleting decomposition became prominent. According to media reports, lobsters then crawled ashore "gasping for oxygen." It bears keeping in mind that natural nutrient inputs may eventually appear to be ‘too intense‘ if the marine food web is drastically weakened. And signals may appear that are indistinguishable from eutrophication, except for their distance from anthropogenic inputs. One media report of the events off Africa is posted online at http://news.nationalgeographic.com/news/2002/01/0131_020130_tvstinkyatlantic.html) (e) Increased growth of filter feeders. It has frequently (maybe consistently) been noted in areas of grossly obvious nutrient overload that filter-feeding organisms that thrive on particulate organic matter, become relatively more dominant in the environment. (Schramm and Nienhuis, 1996) In temperate intertidal zones these are commonly found to be mussels and barnacles. These organisms are good indicators that high nutrient availability persists year round, since individuals commonly live for several years. In an environment with a generally declining level of overall nutrient cycling, however, a decline in the growth of these organisms will ultimately be expected. The ‘filter feeder test’ for eutrophication is therefore unlikely to give false positive results and possibly represents the single most valuable test for the syndrome. Research on marine nitrogen cycling has indicated that: "Low N-loading rates tend to produce systems in which biomass is dominated by benthic plants and their associated predators, whereas high N-loading rates favor dense plankton concentrations and a benthic community dominated by filter feeders." (Carpenter and Capone, 1983) This observation suggests the shifts that would be predicted to occur if an environment were to shift from a relatively high to a lower N-loading regime (an increase in seaweed and a decrease in filter feeders). It also seems to indicate that filter feeders represent a much stronger test for "high N-loading rates" than does macroalgae in general. A close look at macroalgae is warranted nevertheless. (f) Decreased growth of large perennial macroalgae. Significant declines in the larger seaweeds, specifically the kelps and fucoids, have been noted to occur in many marine areas that have received marked increases in terrestrial-source nutrient input. Some of the best known examples are in the Baltic Sea. (Schramm and Nienhuis, 1996) One common observation in these areas is that the fucoid belts no longer extend to the depths that they did previously. The reason for this is believed to be the decreasing light availability in the deeper waters caused by increased shading by the dense growth of phytoplankton. And this is entirely plausible. However, surprisingly perhaps, a situation of declining nutrient availability to macroalgae would also predictably induce a loss of the lower part of the natural range of these plants. The deepest living plants adapt to the lower availability of light by accumulating a greater concentration of light-harvesting pigments. (Lobban and Harrison, 1994) Nitrogen is essential for the formation of the pigments, so the deepest living specimens have naturally a higher nitrogen requirement that plants situated in areas with greater light availability. And therefore, the deepest plants are also likely to disappear first in the case of systemic nutrient loss. A useful signal exists however, to distinguish between the two distinctly opposite possibilities for the disappearance of macroalgae from the deeper areas. In the case of eutrophication/shading, the marginal, stressed plants should not give signals of nutrient shortage, but of intensification of pigmentation before tissue breakdown due to inadequate light. Uptake of nutrients should not be at the root of the problem in these cases, as it is a given that nutrients are present in excessive amounts in these environments. In various species of macroalgae, differing signals may be checked for, based on the differing internal physiological processes in the plants. Fucoid seaweed species, for instance, have a very high demand for nutrients at the growing tips, and they have the capacity to translocate nutrients and products of photosynthesis from the mature, non-growing tissues to the actively growing tips. (Lobban and Harrison, 1994). A signal of nutrient deficiency in these plants, beyond the possible occurrence of lower degrees of pigmentation, would quite plausibly be an extreme draining of the reserves in the mature tissue to support the new growth at the tips. A pattern of extreme withering and loss of mature tissues in Fucus species has been observed in plants growing along the unpolluted Atlantic coastline of Nova Scotia (see the seaweed articles on this website, especially this one which illustrates the pattern of accelerated loss of mature tissue). This pattern has also been commonly seen in other areas (New England, the U.K., the North Sea…personal communication from members of the Algae-L listserver). This seems to be an important signal, the presence of which should help determine whether the problem faced by the seaweed is ‘too much’ or ‘too little’ nutrient availability. (g) Heavy growth of smaller, short-lived macroalgae. Thin, sheet-like macroalgae, along with many filamentous algal forms, are commonly noted to thrive in heavily nutrient-polluted estuaries, and have come to be considered "symptomatic" of eutrophication. (Schramm and Nienhuis, 1996) This test appears to have high sensitivity but very low specificity, since the algal types involved naturally occur across very wide ranges, including the naturally oligotrophic tropical waters. Very adaptable, these "opportunistic" algal forms can take advantage of short nutrient pulses (as may occur seasonally in a starved temperate zone). They can also quickly colonize habitat that has become available due to the disappearance of longer-lived organisms, and can benefit from the reduction of longer lived grazing animals which will predictably also occur in a starved system. Ulva, Enteromorpha and Cladophora species are all too often taken to offer proof of eutrophication by the fact of their very presence, but the specificity of this finding to genuinely nutrient-overloaded waterways is very low. Without exception, these plants have very fine structures and resulting high SA:V ratios, a plant morphology that gives a natural advantage in low nutrient conditions. (It is possible that biochemical analysis of some of these plants may become a useful indicator, but a simple presence/absence test gives virtually no useful information for the purpose of distinguishing nutrient overload from nutrient deficiency.) From this discussion the conclusion emerges that it is surprisingly difficult to distinguish between a "nutrient-overloaded" and a "nutrient-starved" marine system. Many of the common tests used today are prone to giving false positive results. The most important point of distinction probably lies in the observation that the starved system (in temperate zones) will only give false positive signals of nutrient overload on a seasonal basis. In a temperate area where many or all of the symptoms associated with eutrophication persist year round, and include especially a relative dominance of filter feeders, a genuine problem of nutrient overload undoubtedly exists, especially if the area is situated near an obvious source of concentrated terrestrial input. If the signs appear only on a seasonal basis however, or if only one or two of the less specific indicators is noted, a diagnosis of eutrophication cannot be made with any certainty. High nitrogen, elevated chlorophyll, increased turbidity, dominance of filter feeders, and hypoxia appear to be the strongest true indicators (all of these have higher specificity than the simple presence or absence of particular species of macroalgae) and if these tests are positive throughout the entire year, this probably qualifies as the "gold standard" diagnostic test for harmful degrees of anthropogenic nutrient input in marine systems. Are subtle, harmful degrees of eutrophication negatively affecting coastal waters? It is doubtful if this could occur, since the living marine ecosystem is well designed to deal with, and even benefit from, smaller pulses of nutrient input from terrestrial sources as well as those resulting from natural upwelling patterns. "Subtle" effects of eutrophication on coastal waters may unfortunately be indistinguishable from the effects of systemic starvation of coastal waters…and if researchers are only seeking eutrophication, they may mistakenly believe that that is what they have found… (4) CLIMATE CHANGE Changing climate variables can cause changes in levels of marine productivity. Specifically, declines in the "carrying capacity" of ecosystems is recognized as one potentiality. (Beamish, 1995) This has been convincingly proven, especially so in the Eastern Pacific ocean, where predictable shifts in fisheries productivity have been related to ENSO patterns. These fluctuations may, however, be superimposed upon a subtle underlying theme of steady background decline due to nutrient loss. In any case, the distinction between "climate change" and "systemic starvation" can probably best be made in areas not significantly affected by climate change. The Northwest Atlantic ocean presents a good example. Dramatic collapses have occurred in fish stocks that simply have not been accompanied by any impressive degree of climate change. In analyzing the reasons for the collapse of the Northern cod stock, Hutchings and Myers convincingly rejected the hypothesis that it might have been triggered by climate change. (Hutchings and Myers, 1994) General predictions of the effects of climate change on marine populations also include the thought that species may expand their ranges poleward. Range shifts occurring in many species today are not always poleward. A good example is the Northern cod, where the remaining concentrations of these fish are now found in the parts of their former range that have the highest natural nutrient availability (inshore in a few bays of Newfoundland, in the Northern Gulf of St. Lawrence, and on Georges Bank). Incidentally, none of these cod populations are showing any convincing signs of "rebuilding." Poleward range shifts have been noted as well though. One well documented study of a western North American intertidal community demonstrated, over a period of 70 years, a significant decline in species classed as relatively "northern" and a corresponding increase in "southern" types. (Barry et al, 1995) Regarding this change though, it bears pointing out that the "southern" types might be more naturally adapted to lower nutrient regimes as well as to higher temperatures, by comparison to the "northern" types. Teasing apart the relative importance of the possible causes of these community shifts may be difficult. (5) NATURAL PREDATOR IMBALANCE People in some areas are convinced that natural predators are harming fish stocks, and preventing their rebuilding. Examples are the fishermen and scientists in Atlantic Canada who insist that a seal cull is necessary to allow the cod to grow out of the "predator pit" that they believe the seals holding them in. Similarly, it is believed by some in Japan that whales are harming the fish stocks there. Differentiation between a starving ecosystem and an overabundance of natural predators can be aided by checking two important parameters of the prey population. The first is the growth rates and physical condition of the largest prey individuals. If these are found to be in poor condition, population starvation must be suspected. If the largest prey fish are in high condition, however, overexploitation by natural predators might be a possibility (although overexploitation by humans is a more likely scenario since the ecosystem has checks and balances built in to avoid the accumulation of an overabundance of any natural marine predatory species). The other factor that must be assessed is the food supply available to the prey fish population. For most fish, the abundance of zooplankton will be a significant indicator of their food availability. If a given level of zooplankton has been associated in the past with a greater growth of the prey fish, then overexploitation by predators might be limiting the growth of the fish population. If, however, zooplankton levels are at historic lows, then systemic starvation may be the limiting factor on the growth of the fish. And zooplankton is clearly declining in many parts of the world ocean. (Northwest Atlantic: DFO, 2001, California: Roemmich and McGowan, 1995, North Sea: Parsons et al, 1984). (6) ULTRAVIOLET RADIATION Ultraviolet radiation occurs naturally and is capable of causing damage to many organisms, plants and animals alike. In recent decades ultraviolet radiation has increased measurably, particularly at the global poles, and the question often arises of whether this increase might be causing damage to marine life. This is theoretically possible, but a few arguments can be made to show that increasing UV radiation is probably not a major cause of the widespread declining trends in marine life. First, the increasing intensity of UVR has been concentrated at the poles of the planet. Declining patterns in tropical marine life are, however, as impressive as those that have occurred in the temperate and polar regions. (Jackson et al, 2001) Secondly, it will need to be determined at which point in the food web that the radiation damage is being done. Superficially, at least, UVR does not appear to be inhibiting the growth of the phytoplankton, since satellite pictures are showing increasing degrees of "greeness" in many ocean areas. The level of the zooplankton - this is definitely dropping, and it seems as if the damage to the web is occurring on this level. Zooplankters might be naturally vulnerable to damage by UVR, but to quite an extent they follow a behavior pattern of avoiding intense sunlight. Zooplankton are known to undertake substantial daily vertical migrations in the water column. The typical pattern is to rise to the surface layer at night to feed on phytoplankton, and then to swim down to much deeper levels during the day. Most of their daylight hours are therefore spent more or less ‘in the shade.’ Lastly, it appears that the declining trend in marine productivity has been going on for a much longer time than has the rising trend in UVR. The maximum sizes obtained by many marine species, for instance, seems to have been dropping for many more decades than UV has been rising. Fisheries had already experienced significant declines before humans began releasing CFCs into the atmosphere. (Mowat, 1984, Jackson et al, 2001) UV radiation might be inflicting damage on some marine organisms today, but it is not credible as a major causative factor that is forcing the general decline. A plausible "cause" must be seen to precede the appearance of the presumed "effect."
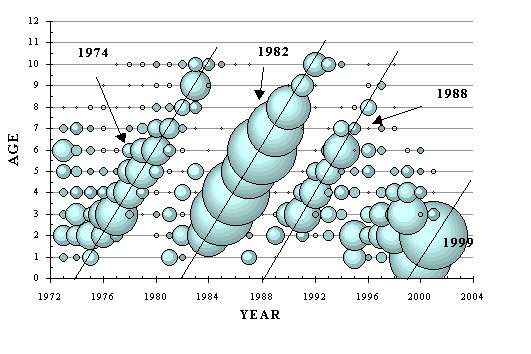
Historical summary: Extremely old, life in Earth’s ocean has experienced a few ups and downs before this time. On past occasions marine life has recovered from mass extinction events, albeit this recovery has been very slow by human time scales. The presently existing assemblage of marine organisms evolved over recent hundreds of millions of years, and has seemingly maintained a fairly stable composition and level of productivity for thousands of years prior to the present time. Over the last 1000 years, predation on marine life by terrestrial mammals has risen to an unprecedented level. Virtually all larger marine species have been targeted by this predation, although there was a tendency at first to target the very largest species and those organisms living at highest trophic levels. Massive declines have occurred in the populations of targeted species, which has also affected many unexploited species due to altered patterns of species interactions. (Jackson et al, 2001) Major physical findings today, with a consideration of a possible diagnosis of systemic starvation: Ideally considered in an initial assessment perhaps, is the Canadian section of the Northwest Atlantic ocean, a large marine ecosystem that has not experienced a significant degree of climate change to date, but has been the subject of a substantial degree of scientific investigation. Major changes have occurred in the biota of this part of the world ocean, and it can be demonstrated that these changes are essentially consistent with the starvation model and anticipated ‘homeostatic’ responses. Significant evidence includes: - A decline in the maximum sizes of fish has occurred. (e.g. Pauly et al, 1998) - A "down the web" shift in species composition has occurred (areas formerly dominated by fish are now dominated by crustaceans - this is well known, but a more subtle "down the web" shift has also occurred in unexploited species such as those occurring in the intertidal zone - see the articles on barnacles and seaweed posted on this website.) - Ranges of some species have visibly contracted into the areas where nutrient availability is naturally relatively higher (as discussed in earlier sections of this report). - An increasing frequency and extent of harmful algae blooms has occurred. (Lassus et al, 1995) - Declining age and size at maturity has been noted in all species for which this data has been collected. Besides groundfish and pelagic fish, this trend includes crustaceans such as lobster, crab and shrimp. (DFO…all recent stock status reports are available online at www.dfo-mpo.gc.ca/csas/csas/ ) - An increasing intensity and duration of the spring phytoplankton bloom has been recorded in recent years. (DFO, 2000c, DFO, 2001) - A declining trend in zooplankton abundance has occurred. (DFO, 2000c, DFO, 2001) The declines in marine life in Atlantic Canada have been largely attributed to "overfishing," therefore this is the differential diagnosis that needs to be most carefully made. Three fish species will be considered in more detail: Northern cod, capelin and Atlantic mackerel. (1) Northern cod. Although difficult to ascertain from early fishery records (which mostly have included only tonnage landed), it appears that the decline in the maximum size of cod occurred over many decades. The decades during which fishery statistics included biological data (approx 1960 - present) roughly indicate that a steady decrease in the maximum age and size of cod occurred throughout this time. But was that the result of direct fishing pressure applied to the cod stock, or to a lowering of the scope for growth of cod in the ecosystem? Records on the cod stock include growth indices (although not commonly condition factor) for individual age cohorts, and these records show declining growth indices which occurred, at the outset, more steeply in the oldest ages and not at all in the younger ages. This trend immediately preceded the disappearance of the older ages of fish from the data tables. (Lilly et al, 1999) Both the precipitous nature of the population collapse, and the failure of the cod stock to recover under a prolonged fishing moratorium, are suggestive of systemic starvation or "bottom-up control" as a major underlying factor that has forced this decline. Indices of cod growth continue to decline in Atlantic Canada, seeming to indicate a further steady lowering of the scope for fish growth and productivity of this ecosystem. (Although the groundfish fisheries have been scaled back greatly in recent years, they have not been eliminated, and large amounts of marine biomass extraction still occurs in the form of crustaceans - a practice that "might" also further hamper nutrient cycling and productivity of the system… "if" fishing is implicated as a cause of the problem.) Although the sequence of events that has occurred with the Northern cod is consistent with the starvation model, this stock has been subjected to centuries of intense fishing, and the suspicion will remain that the cod reacted ultimately only to the recent cod fishery, rather than to a more subtle and longer-term ecosystem effect (which ‘might’ still be an effect of fishing, but via a longer-term and slightly indirect route). If the forcing mechanism that caused the cod collapse was a relentless lowering of scope and production in the overall ecosystem, then lightly exploited and unexploited species in the same area must also show the same trend. Unfortunately, detailed time-series biological records have not been kept on unexploited species (with the partial recent exception of plankton), but a similar pattern of decline can be demonstrated in two lightly fished commercial species, capelin and mackerel. (2) Capelin. This small, cold water pelagic "baitfish" is a major prey species for cod, other predatory fish, marine mammals and seabirds. Fisheries exploitation of capelin has been at such a low level compared to natural predation, that it is believed that no biological effects could have been exerted on the capelin stock by the capelin fishery per se. (Carscadden et al, 2001) Unexpectedly, however, capelin has declined as its major predator (cod) has disappeared. Once a vast population of capelin existed on the offshore Newfoundland shelf, but now only a remnant remains there. (acoustic survey, DFO 2000a, Carscadden, 2001) The remaining concentrations of capelin, as with the cod, are now located in nearhore waters. Declining biomass aside, have the signals of population starvation appeared in the capelin stock? Recent assessments on capelin in Atlantic Canada have recorded mature fish aged 2 - 4 years old (DFO, 2000a, Carscadden, 2001) while older references describe mature capelin as commonly living to age 5 years, with a maximum observed age of 7 years. (Leim and Scott, 1966) The maximum age of capelin appears to have been lowered. In recent decades scientists have documented a decrease in the average size of capelin, as well as a population shift towards a higher proportion of younger fish in the spawning population. The average age at first maturity is also dropping significantly. And the reasons for these shifting trends are "unknown." (DFO, 2000a) Data on condition of capelin is sketchy beyond the observation that they are generally smaller now than they have historically been known to be. The specific signal of declining condition in the older ages of capelin cannot be detected although it may certainly be part of the general decline in size of fish. Age contraction of the stock (the single early signal of food limitation) and declining age at maturity (a hypothetical signal of declining productivity), however, seem consistent with the possibility of an increase in bottom-up control of capelin. (3) Atlantic mackerel. A migratory schooling pelagic fish that historically lived to 20 years, the Atlantic mackerel ranges from Newfoundland to North Carolina. The stock component appearing in Canadian waters has experienced a rapid contraction of its age structure over the last few years (based on assessment of commercial catches, DFO, 2002). In recent years, directed fishing pressure on the mackerel stock has been considered to be relatively light, so "overfishing" can plausibly be ruled out as the cause of this change. It has been believed by some scientists that exploitation rates on mackerel could safely be increased (Overholtz, 2000 (NMFS)). Yet, at least on the Canadian side, mackerel stock assessments are revealing alarming signals of a decline, one which cannot be clearly related to the mackerel fishery itself. For example, estimates of spawning biomass have dropped substantially "…between 1993 and 1996 inclusive, the spawning biomass of mackerel in the Gulf apparently dropped from 936,000t to 126,000t, a difference of 810,000t. During the same period, reported landings for eastern Canada were only 85,376t…" (DFO, 2002) But spawning biomass is not the critical signal when assessing for the possibility of a starvation-induced population decline. What is the recent trend for the age structure of the Atlantic mackerel stock?
Trends in age at maturity and condition factor data by age cohort have not been reported in the scientific assessment of the mackerel stock, but the essential signal seems to be loud and clear: The only early warning signal of the onset of population starvation is the loss of the older, larger individuals. The big mackerel are not being harvested by fishermen, it appears possible that they are now being "harvested" by the ocean herself. In all likelihood the large mackerel are now dying of starvation and their nutrients are being recycled in the larger systemic effort to maintain stability of the plankton and the supply of dissolved nutrients in seawater. The trajectory of the recent decline in the age of mackerel is so steep that stock extinction appears to be imminent. This collapsing trend echoes the steep contraction in age structure that accompanied the collapse of Northern cod a decade ago. The cod however, has so far managed to continue to linger and survive in some near shore refuges. This coping mechanism will not likely work for the mackerel stock however, due to its long migration pattern and affinity to warmer waters. A few mackerel may manage to linger at the continental shelf break, where upwelled nutrients and warmer water may sustain them for a while, but it is very doubtful that a successful spawning outcome for mackerel could occur without following its traditional pattern of inshore migration. The prognosis for Atlantic mackerel now looks very poor. For how long has the Atlantic mackerel been affected by the "lowering of the ceiling?" Long term data is sketchy, but the insidious decline and loss of the larger fish appears to have spanned many more years than just the last couple of decades. Older scientific references to mackerel in Atlantic Canada state that they "rarely" were seen at sizes above 4 pounds in weight and 22 inches in length. (Leim and Scott, 1966) That describes what would be an unbelievably large mackerel today. Length and weight data on mackerel collected by DFO scientists from the 1970s till the present indicate that no significant numbers of mackerel in those years have grown above 2 pounds in weight or 16 inches in length. (DFO, 2002) (Incidentally, regarding DFO's Fig 6, "Canadian catch at age" for mackerel, a lesser contraction in the age structure of the stock was apparent in the 1970s. The extreme concentration of biomass in the lowest age cohorts did not occur at that time, however. The 1970s age contraction in mackerel was a reflection of the effects of "overfishing" which occurred at the time as mackerel were heavily exploited offshore by a large foreign fishery. The ‘scope for growth of mackerel’ in the ecosystem had not been lowered so far in the 1970s as it is now however, and the release of the foreign fishing pressure in 1977 resulted in a prompt rebounding of the age structure of the mackerel stock. All of these elements are the same as the tales of the Northern cod and the other groundfish: they were overfished in the 1960s and 1970s, but promptly rebounded when fishing pressure was relaxed. Then a few decades later these same fish stocks were overtaken by a sudden collapse from which they remain unable to recover. This illustrates, in the first part, the effects of "stock overfishing" on individual species and in the second part, the effects of "ecosystem overfishing" on all of them.) At this point, the history and current physical findings offer grounds for a strong suspicion that starvation is increasingly limiting the potential for growth of all marine life in Atlantic Canada. Total primary productivity in this ecosystem appears to be dropping, as indicated by the steady and increasingly acute loss of all larger fish. These observations from Atlantic Canada are consistent with findings elsewhere in the world ocean that seem to point towards the same conclusion: a broad systemic lowering of "productivity" rates, increasing "bottom-up control" or "mounting starvation," whichever phrase you prefer… Observations from other parts of the world ocean include a few interesting details: - The problems that have plagued the tropical corals in recent years, epidemics of disease and mass bleaching and death during warm periods, these developments offer a picture that is indistinguishable from the scenario that would be predicted to unfold "if" increasing degrees of bottom-up control were applied to tropical ecosystems. (See coral report on this website.) The increasing prevalence of cyanobacteria in tropical reef communities, including their direct implication in some emerging coral diseases, is consistent with the general expectation that, under increasingly nutrient-limited conditions, cyanobacterial growth (and nitrogen fixation) will increase. And the overgrowth of coral reefs by macroalgae is consistent with a shift towards lower N-loading rates, as predicted by the observations of Carpenter and Capone (1983). - The declining trend in sizes of marine fish and their ages at maturity has been well documented in the North Pacific as well as in the North Atlantic ocean. But an interesting aberration from the pattern has also been recorded across the North Pacific. The size of five species of Pacific salmon, from North America and Asia, has shown a steady and very substantial decline in average size over recent decades (Bigler et al, 1996, Helle and Hoffman, 1995). But, instead of showing the lowered age at maturity, as in the marine fish stocks, Pacific salmon clearly show the opposite tendency. Age at maturity is significantly increasing in Pacific salmon stocks (both North American and Asian) that have been examined for the trend. (Helle and Hoffman, 1995) How do these changes in salmon compare to the predicted trends in a "starving ocean" and the hypothesis that declining age at maturity represents a systemic effort to enhance zooplankton? Since salmon are not marine spawners, perhaps the imperative to produce spawn at a younger age in conditions of lowered zooplankton is not felt by these species. The spawn that salmon produce does not play a part in marine zooplankton. The size decline in Pacific salmon appears certainly to reflect a decline in their feeding opportunities, which is consistent with a decline in ocean productivity. So salmon compensate now by spending more years at sea in order to accumulate enough stored energy before attempting to ascend the rivers. And they are tackling the rivers with far lower energy reserves than they did in the past. For example, populations of chum salmon in Alaska and Washington states were observed to experience an average body weight decline of 46% between 1976 and 1991. (Helle and Hoffman, 1995) Salmon appear to be compensating on two fronts for the declining feeding opportunities in the North Pacific, they have steadily increased their age and decreased the energy that they store for their spawning migration. One or the other of these variables may be approaching the physiological limit for survival of these fish. It is difficult to guess just how close these species are "to the wall" at present, but in river pre-spawning mortality due to low energy reserves and high parasite loads seems to increasingly be an issue with some salmon runs. Warmer water aggravates these deaths, but may not be the real root of the problem. As with the sudden collapse predicted for the oysters in the model, and the "crashes" experienced by the Northern cod (and now seemingly the Atlantic mackerel) in Atlantic Canada, a further slight reduction in marine productivity of the North Pacific may suddenly eliminate a large portion of these salmon. Biomass and abundance estimates will not be the biological reference points that will signal the approaching collapse of Pacific salmon. Monitoring of the base of their food web, not the phytoplankton but the zooplankton, will be the single best indicator - and if it continues to drop, the immediate danger to the salmon may very suddenly become apparent. A final observation can be made regarding changing trends in age and size at maturity of marine fish, parameters which are widely declining. A model of "plasticity for age and size at sexual maturity" in fish has been described in the literature. (Stearns and Crandall, 1984) This model predicts several different trajectories along which these parameters may change, depending on which types of environmental stressors force the changes. If environmental conditions cause an increase in adult mortality but no change in juvenile mortality (as in the starvation model of Powell et al), then the model of Stearns and Crandall clearly predicts that organisms will mature earlier and at smaller sizes. The consistency of these general predictions with the declining trends widely observed today in the ages and sizes at maturity of marine fish (including lightly exploited species), further supports the hypothesis that ever-increasing bottom-up control, or ‘starvation,’ is forcing the changes.
If humans prefer to see marine life continue to persist as we have come to know it in recent centuries, instead of witnessing an abrupt "regime shift" to a less productive system dominated by bacteria, toxic phytoplankton and jellyfish, then we urgently need to change our approach. We will need to stop aggravating the problem and find a way to asssist the natural healing mechanisms within the patient, the ‘internal organs’ that produce and support the zoopankton assemblage. If we prefer to carry on as usual and watch ‘natural events’ unfold, then it is time now to brace ourselves from some very nasty surprises. Major ‘internal organs’ involved in the sustenance of marine zooplankton are the entire group of organisms that produce pelagic spawn. This fact has been largely unrecognized in scientific analyses of the workings of the marine ecosystem. Recognizing this fact now should lead to another urgent rationale to stop bottom trawling, since this practise destroys key benthic organisms that are important in nutrient cycling far beyond their role in providing forage for bottom feeding fish. But stopping bottom trawling will not be enough to halt the declining trend in marine productivity. Any continued marine biomass extraction that is not appropriately replaced will further exacerbate the ongoing problem. And ‘the patient’ has already been brought very low. (Mowat, 1984, Jackson et al, 2001) If any "treatment" is to be attempted besides stopping fishing, it probably should be in the form of a solid food subsidy that can be directly consumed by benthic scavengers and fish, something that can be eaten directly by organisms that produce pelagic spawn. The simple addition of dissolved nutrients to seawater will not result in those nutrients being taken up and recycled as effectively in the larger web. (This particular experimental treatment has already been well tested, and the dearth of marine life in polluted estuaries attests to its failure.) Currently a major international scientific research effort is directed towards monitoring the ocean to discern the ecosystem effects of climate change (JGOFS). Detailed investigations are being made into the question of how climate will affect marine food webs and marine productivity in the future. What is urgently needed however, and long overdue, is an intensive and objective scientific inquiry into the effects on marine productivity and marine food webs that have already been induced by centuries of fishing. Marine biology now needs to make a quantum leap in thinking. A quick divorce from the commercial fishing industry and single stock management activities is necessary, and a wholehearted commitment to the ideals of "conservation" and "ecosystem health" is imperative for the branch of science charged with the responsibility of safeguarding the ocean, the original ‘lifeblood’ of this planet…To do anything less is the equivalent of continuing to use medical doctors who persist in trying to heal a sick patient by "bloodletting," a primitive and lethal practise long ago abandoned by scientists as they gradually became enlightened, over centuries, into the basic principles that govern the maintenance and restoration of human health. Trial and error in the early days of medical science often resulted in the unanticipated death of the patient. Many things in medicine were not at all as they first appeared to be, and the true functions of many mysterious body parts were only discovered in the later stages of the study. Many are still not understood. The current level of misunderstanding and uncertainty in marine science is not the fault of today’s marine scientists, it simply reflects how young their science is, and how large and difficult to understand their ‘patient’ is… Unfortunately, marine science’s first ‘patient’ will also be their last. The consequences of a single fatal error will be permanent and far-reaching, not only for marine life but for a great number of terrestrial forms as well. It is clear that biological systems can withstand and adjust to various levels of different stressors. They have a natural resiliency. But it is also clear that any of these systems can be "pushed too far," and will eventually reach a critical point where major and irreversible changes take place "suddenly." Earth’s ocean ecosystem is showing multiple signs now that the human fishing pressure is too intense. "Decompensation" will result in a sudden irreversible change. It is not possible to predict exactly how soon the permanent ‘downshift’ will occur, beyond noting that it appears likely to happen ‘soon.’ The problem of the ailing ocean is now at a critical point, and marine biology urgently needs to seek helpful insights from every and any field of human knowledge that might possibly assist in putting the pieces of the puzzle together and finding the best solution.
Barry, J. P., C. H. Baxter, R. D. Sagarin, and S. E. Gilman. 1995. Climate-related, long-term faunal changes in a California rocky intertidal community. Science 267:672-675. Bates, Nicholas R. 2001. Interannual variability of oceanic CO2 and biogeochemical properties in the Western North Atlantic subtropical gyre. Deep-Sea Research II 48(2001):1507-1528. Beamish, R. J. (ed). 1995. Climate change and northern fish populations. Can. Spec. Publ. Fish. Aquat. Sci. 121:739p Berger, W. H., V. S. Smetacek and G. Wefer (eds). 1989. Productivity of the Ocean: Present and Past. John Wiley and Sons. Bigler, B.S., D. W. Welch, and J. H. Helle. 1996. A review of size trends among North Pacific salmon (Onchorhynchus spp). Canadian Journal of Fisheries and Aquatic Sciences 53:455-65 Carscadden, J. E., K. T. Frank and W. C. Leggett. 2001. Ecosystem changes and the effects on capelin (Mallotus villous), a major forage species. Can. J. Fish. Aquat. Sci. 58:73-85 (2001). (online at http://www.nrc.ca/cisti/journals/sample/f00-185.pdf ) Carpenter, Edward J. and Douglas G. Capone. 1983. Nitrogen in the Marine Environment. Academic Press. DFO, 2000a. Capelin in Subarea 2 + Div. 3KL. DFO Science Stock Status Report B2-02 (2000). DFO, 2000b. State of Phytoplankton and zooplankton in the Estuary and northwestern Gulf of St. Lawrence during 1999. DFO Stock Status Report C4-18 (2000). DFO, 2000c. State of Phytoplankton, Zooplankton and Krill on the Scotian Shelf in 1998. DFO Stock Status Report G3-02(2000). DFO, 2001. Chemical and biological oceanographic conditions 2000 - Maritimes region. DFO Science Stock Status Report G3-03 (2001). DFO, 2002. Atlantic Mackerel of the Northwest Atlantic - Update (2001), DFO Science, Stock Status Report B4-04 (2002) Godo, O. R. and T. Haug. (? date - this document describes observations made in Norway) Growth Rate and Sexual Maturity in Cod (Gadus morhua) and Atlantic Halibut (Hippoglossus hippoglossus) J. Northw. Atl. Fish. Sci., Vol 25: 115-123. Goni, Raquel. 2000. Fisheries Effects on Ecosystems. In Seas at the Milennium: An Environmental Evaluation (Edited by C. Sheppard). Elsevier Science Ltd. Helle, J. H. and M. S. Hoffman. 1995. Size decline and older age at maturity of two chum salmon (Oncorhynchus keta) stocks in western North America, 1972-92. In Climate change and northern fish populations. Edited by R. J. Beamish. Canadian Special Publication of Fisheries and Aquatic Sciences. 121:245-260. Hutchings, Jeffrey A. and Ransom A. Myers. 1994. What Can be Learned from the Collapse of a Renewable Resource? Atlantic Cod, Gadus morhua, of Newfoundland and Labrador. Can. J. Fish. Aquat. Sci. Vol 51:2126-2144. Jackson, Jeremy B. C. 2001. What was natural in the coastal oceans? Proc. Natl. Acad. Sci. USA, Vol 98, Issue 10, 5411-5418, May 8, 2001. Jackson, Jeremy B. C. et al. 2001. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 293:629-637. Kaiser, Michael J. and Sebastiaan J. de Groot. 2000. Effects of Fishing on Non-Target Species and Habitats, Biological, conservation, and socio-economic issues. Blackwell Science. Kasyanov, V. L. 2001. Reproductive Strategy of Marine Bivalves and Echinoderms. Science Publishers Inc., USA Kerfoot, W. Charles and Andrew Sih. 1987. Predation, Direct and Indirect Impacts on Aquatic Communities. University Press of New England. Lassus et al. 1995. Harmful Marine Algal Blooms. (International Conference on Toxic Marine Phytoplankton (6th: 1993, Nantes)). Lavoisier, Intercept Ltd. Lilly, G. R., P.A. Shelton, J. Brattey, N. Cadigan, E.F. Murphy and D.E. Stansbury. 1999. An assessment of cod stocks in NAFO divisions 2J+3KL. Canadian Stock Assessment Secretariat Research Document 99/42 (available online at www.dfo-mpo.gc.ca./csas/csas/ ) Lobban, Christopher S. and Paul J. Harrison. 1994. Seaweed Ecology and Physiology. Cambridge University Press. Mowat, Farley, 1984. Sea of Slaughter. Toronto: Bantam Books. Overholtz, William. 2000. Atlantic Mackerel. (New England stock report online at http://www.nefsc.nmfs.gov/sos/spsyn/pp/mackerel/ ) Parsons, T. R., M. Takahashi and B. Hargrave. 1984. Biological Oceanographic Processes (3rd edition). Pergamon. Pauly, Daniel, Villy Christensen, Johanne Dalsgaard, Rainer Froese, and Francisco Torres Jr. 1998. Fishing Down Marine Food Webs. Science 279:860-863. Powell, EN, JM Klinck, E Hoffmann, EA Wilsonormond, MS Ellis. 1995. Modeling Oyster Populations V. Declining Phytoplankton Stocks and the Population-Dynamics of American Oyster (Crassostrea-Virginica) Populations. FISHERIES RESEARCH 24:(3)199-222, October 1995. Roemmich, Dean and John McGowan. 1995. Climate Warming and the Decline of Zooplankton in the California Current. Science 267:1324-26. Schramm, Winfrid and Pieter H. Nienhuis (eds). 1996. Marine Benthic Vegetation, Recent Changes and the Effects of Eutrophication. Springer. Shaffer, Gary. 1993. Effects of the Marine Biota on Global Carbon Cycling. In Heimann, Martin (ed) 1993. The Global Carbon Cycle. NATO Advanced Science Institutes Series. Berlin: Springer-Verlag. Stearns, Stephen C and Richard E. Crandall. 1984. Plasticity for Age and Size at Sexual Maturity: A Life-history Response to Unavoidable Stress. IN Potts, G. W. and R. J. Wootton (eds) 1984. Fish Reproduction: Strategies and Tactics. Academic Press, London, p 13 - 33. Steidinger, Karen A. and Linda M. Walker. 1984. Marine Plankton Life Cycle Strategies. CRC Press, Inc., Florida. Zhong, Zheng, et al. 1989. Marine Planktology. Beijing: China Ocean Press Copyright ©
Debbie MacKenzie, 2002 |